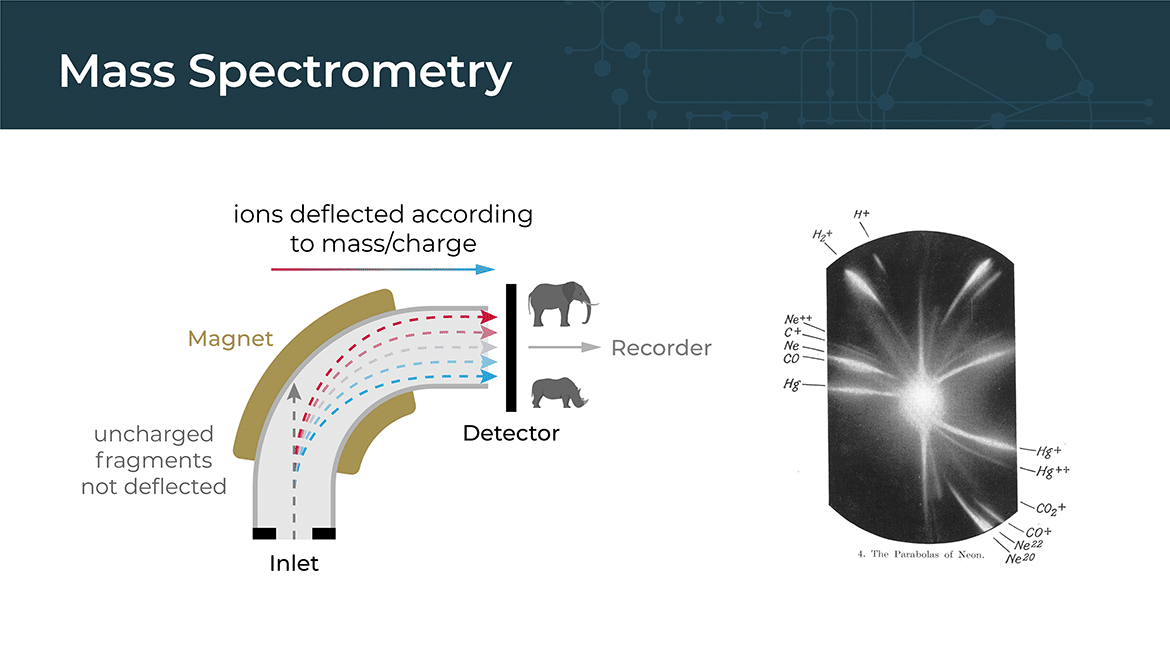
Mass Spectrometry
Mass spectrometry is a powerful analytical technique used to identify and quantify the chemical components of a sample based on their mass-to-charge ratio. It involves ionizing the molecules in a sample and then separating them based on their mass-to-charge ratio using an electric or magnetic field. The resulting data can be used to identify the chemical composition of the sample and to measure the abundance of different molecules.
Mass spectrometry can be used in conjunction with cryo-electron microscopy (cryo-EM) to provide complementary information about the sample being studied. For example, mass spectrometry can be used to identify the proteins or other biomolecules present in the sample, and to determine their molecular weights and post-translational modifications. This information can then be used to help interpret the cryo-EM data and to build a more accurate model of the molecule being studied.
In addition, cryo-EM and mass spectrometry can be used together in a technique called integrative structural biology. In this approach, the cryo-EM structure of a molecule is combined with other structural and biochemical data, such as mass spectrometry results, to build a more complete and accurate model of the molecule. This can provide valuable insights into the function of the molecule and its interactions with other molecules in the cell.
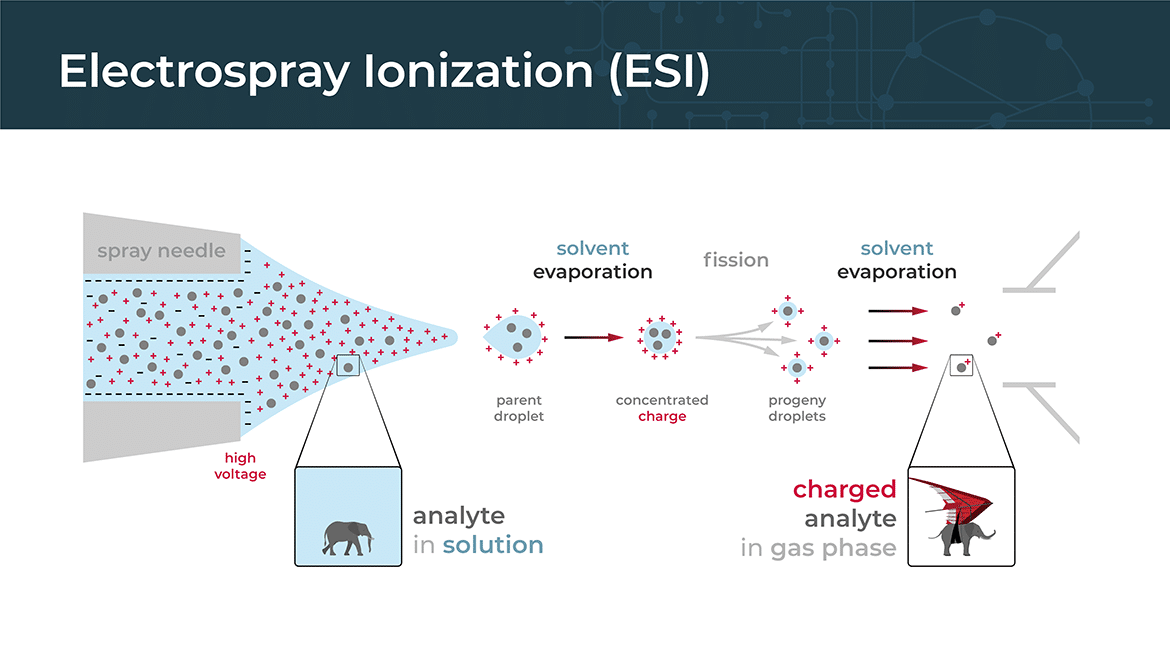
Electrospray ionization (ESI) is a commonly used ionization technique in mass spectrometry for analyzing large biomolecules such as proteins, peptides, and nucleic acids. It involves the formation of charged droplets from a solution containing the analyte molecules, followed by the generation of gas-phase ions from these droplets.
Matrix-assisted laser desorption/ionization
Matrix-assisted laser desorption/ionization (MALDI) is a common ionization technique used in mass spectrometry to analyze large biomolecules such as proteins, peptides, and nucleic acids. In this technique, the sample is mixed with a matrix compound that helps to ionize the analyte molecules when they are hit by a laser pulse.
Matrix landing is a term used in MALDI mass spectrometry to describe the process of transferring the ions generated by the laser pulse from the matrix layer to the gas phase of the mass spectrometer. In this process, the laser pulse vaporizes the matrix compound and the analyte molecules embedded in it, producing a plume of ions that are then drawn into the mass spectrometer by an electric field.
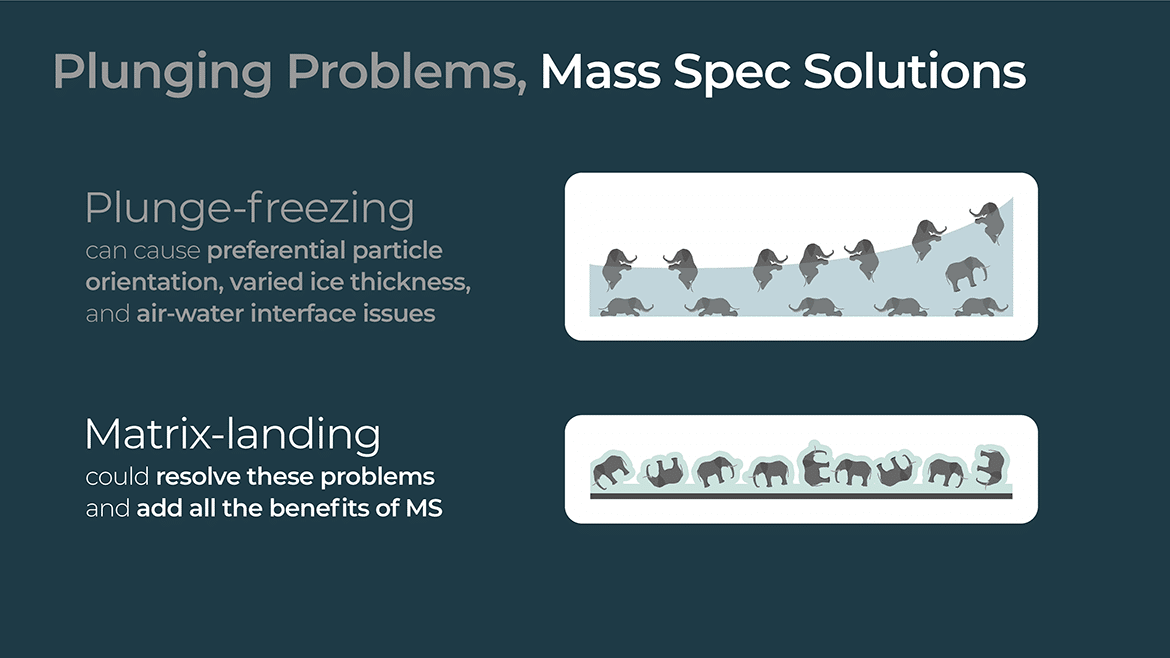
The matrix plays a critical role in this process, as it absorbs the laser energy and helps to ionize the analyte molecules by releasing protons or other cations. The matrix also helps to protect the analyte molecules from being damaged by the laser pulse, as it absorbs much of the energy and dissipates it as heat.
Once the ions have been generated by the laser pulse, they are accelerated by an electric field and focused into a narrow beam, which is then directed into the mass analyzer. The mass analyzer separates the ions based on their mass-to-charge ratio, allowing them to be detected and quantified.
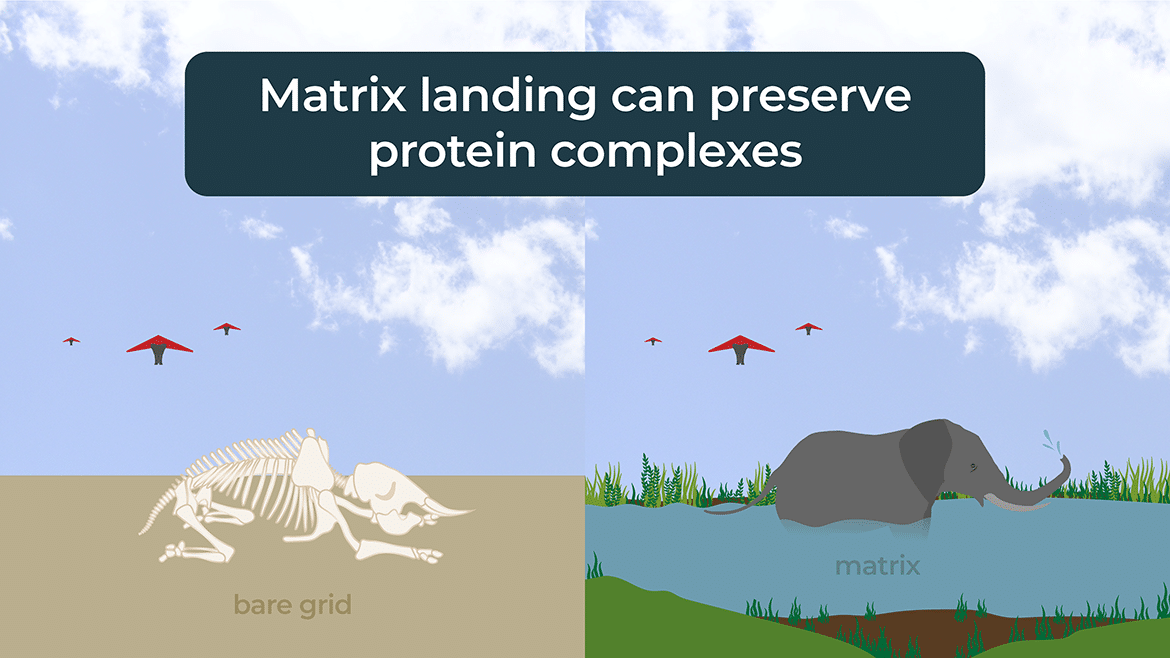
Overall, matrix-assisted laser desorption/ionization (MALDI) is a powerful technique for analyzing large biomolecules, and matrix landing is a critical step in the process of transferring the ions from the matrix layer to the gas phase of the mass spectrometer.