Tag: Thomson Lab
Bioengineered arteries show promise for cardiovascular surgery
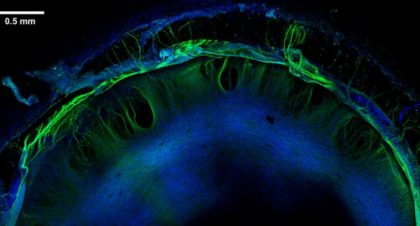
Scientists bioengineered stem cell-derived artery grafts that could advance the field of vascular bypass surgery to combat cardiovascular disease.
Rising Sparks: Marcela Tabima, regenerative biology
Marcela Tabima has focused her fascination with human biology on two major research efforts in the Discovery Building — engineering synthetic arteries for surgery and finding new ways to treat inherited retinal diseases.
All creatures great and small: Sequencing the blue whale and Etruscan shrew genomes
Researchers illustrated that size doesn’t matter when they assembled sequences for two new reference genomes — one from the world’s largest mammal and one of the smallest.
CAR macrophages show promise for cancer immunotherapy treatments
Scientists use stem cells to develop CAR macrophages that can infiltrate and kill tumor cells, showing promise for cancer immunotherapy treatments.
Meet The Lab inspires students with Morgridge Institute research
Classrooms from Wisconsin and beyond explore Morgridge science through new Meet the Lab resources from PBS Wisconsin.
Morgridge alum awarded for “FANTM” limb virtual technology
Former Morgridge Postdoctoral Fellow Finn Kuusisto was awarded for his work developing information technology to improve virtual reality applications that could one day be applied to biomedical devices.
A science trailblazer retires: Stem cell researcher James Thomson’s legacy changed the future of biology
James Thomson helped the scientific world turn its attention to the remarkable stem cells that give rise to all of the building blocks of life. After more than 30 years with UW–Madison and 15 years with the Morgridge Institute, Thomson has announced plans to retire in July 2022.
Thomson Lab alumna gives back in new outreach project
Thomson Lab alumna Shannon Strader returned to her roots at Morgridge to participate in an upcoming outreach feature with PBS Wisconsin Meet the Lab.
Thomson Lab looks to make major health impact with artery engineering project
Stem cell pioneer James Thomson is leading a potentially transformational project to develop a safe and functional cell-based artificial artery that could be pulled from medical inventories and used by vascular surgeons.
Where are they now? Morgridge alumni look back (and ahead) at their careers
A few of our Morgridge alumni shared thoughts on their research experience at the Morgridge Institute, their plans moving forward and their warm shoutouts to some of the people who helped them along the way.
The Power List 2020
via The Medicine Maker
Stem cell pioneer and Morgridge investigator James Thomson was named to the Power List 2020 for his contributions to advancing the field of medicine to save lives and improve the world.
Congratulations to May 2020 Graduates
Congratulations to the graduating students and research staff as they move onward and upward. A few of these students and staff shared about their time at the Morgridge Institute, their accomplishments and their plans for what’s next.
Human developmental clock mimicked in a dish
A Morgridge regenerative biology team has created a first-ever human model for developmental timing: A “clock in a dish” that will help explore mysteries of early human development.
Stem cell scientists clear another hurdle in creating transplant arteries
Scientists at the Morgridge Institute are working toward a dream of creating artery banks with readily-available material to replace diseased arteries during surgery. Recent work highlights highlights a better way to grow smooth muscle cells, putting the science one step closer to that goal.
Stem cells: Where are we going?
Together with the University of Wisconsin–Madison, we look back on 20 years of stem cell research and see where we’re going next. Catch up on what’s happened since James Thomson’s prescient prediction that stem cells “will change medicine, period.”
Collaboration leads to ‘dream’ of artery bank
Just as blood banks are essential to medicine, the Thomson Lab hopes to see the advent of artery banks that give surgeons a better, readily available material to replace diseased arteries. The lab is using pluripotent stem cells to grow the cellular building blocks of the artery — endothelial and smooth muscle cells — and coax them into assembling into arteries that can grow and thrive in a majority of patients.
Out of left field: Thomson looks at discovery and the future of science
James Thomson, the UW–Madison biologist whose stem cell discovery 20 years ago opened fascinating and promising new avenues in science, took time to discuss his thoughts on the breakthrough and what the future holds for the field of regenerative medicine.
New stem cell method sheds light on a tell-tale sign of heart disease
While refining ways to grow arterial endothelial cells in the lab, a regenerative biology team at the Morgridge Institute for Research unexpectedly unearthed a powerful new model for studying a hallmark of vascular disease.
Stem cell advance brings bioengineered arteries closer to reality
New techniques developed at the Morgridge Institute for Research and the University of Wisconsin–Madison have produced, for the first time, functional arterial cells at both the quality and scale to be relevant for disease modeling and clinical application.
Can artery ‘banks’ transform vascular medicine?
The prospect of creating artery “banks” available for cardiovascular surgery, bypassing the need to harvest vessels from the patient, could transform treatment of many common heart and vascular ailments.
Thomson honored for stem cell research legacy
Wisconsin biotechnology advocacy organization BioForward honored two giants in state biomedical innovation — stem cell research pioneer James Thomson and entrepreneur Ralph Kauten — for their scientific and business achievement during its annual summit September 27 in Madison.
Stem cells help predict neural toxicity
A new system developed by scientists at the Morgridge Institute for Research and the University of Wisconsin–Madison may provide a faster, cheaper and more biologically relevant way to screen drugs and chemicals that could harm the developing brain.
James Thomson: Opening access to the cellular building blocks of life
Thomson’s discoveries in human stem cell research at UW–Madison have redefined biomedicine, first with the isolation and culturing of human embryonic stem cells in 1998; then in the development of human pluripotent stem cells from adult skin cells in 2007.
A shift in stem cell research
A team of engineers at the University of Wisconsin–Madison has created a process to improve the creation of synthetic neural stem cells for use in central nervous system research.
Thomson lab lands $2.2 million NIH grant
With a $2.2 million grant from the National Institutes of Health, stem cell pioneer Dr. James Thomson, University of Wisconsin–Madison associate professor of biomedical engineering William Murphy and School of Medicine and Public Health medical informatics professor David Page will lead a team to derive and assemble the distinct cell types found in the human cerebral cortex.
Study shows patient’s own cells may hold therapeutic promise after reprogramming, gene correction
Scientists from the Morgridge Institute for Research, the University of Wisconsin–Madison, the University of California and the WiCell Research Institute moved gene therapy one step closer to clinical reality by determining that the process of correcting a genetic defect does not substantially increase the number of potentially cancer-causing mutations in induced pluripotent stem cells.
Wisconsin, Morgridge scientists excise vector, exotic genes from induced stem cells
A team of scientists from the Morgridge Institute for Research at the University of Wisconsin–Madison reports that it has created induced human pluripotent stem (iPS) cells completely free of viral vectors and exotic genes.
Stem cell pioneer James Thomson to steer regenerative medicine at MIR
The Morgridge Institute for Research, the private, not-for-profit side of the Wisconsin Institutes for Discovery, is announcing the appointment of world-renowned stem cell pioneer and researcher James Thomson as the first member of its multidisciplinary scientific leadership team.